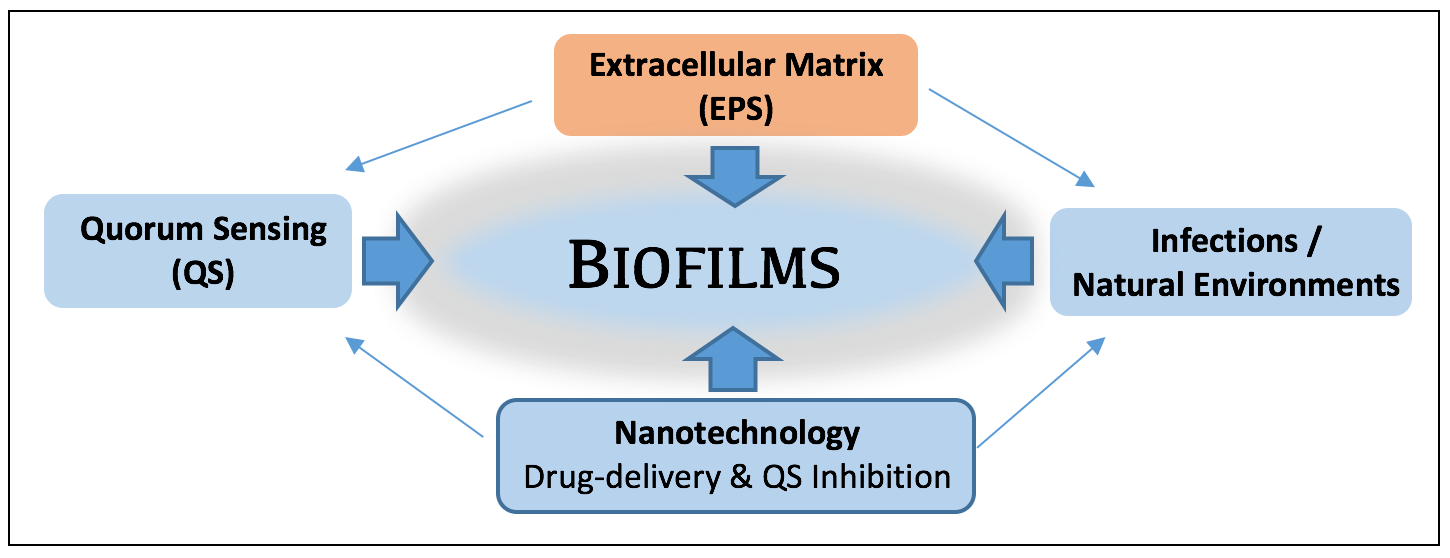
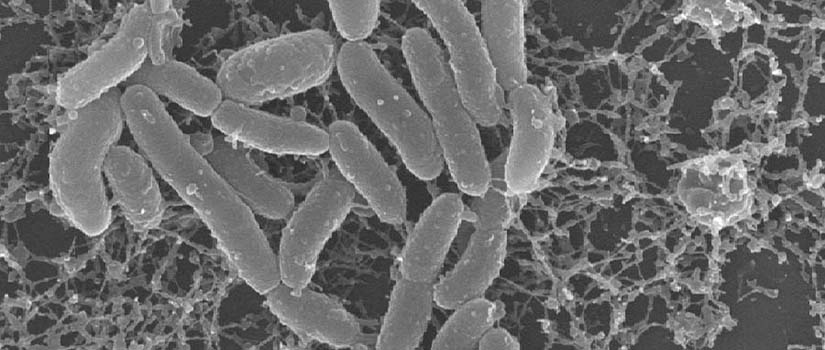
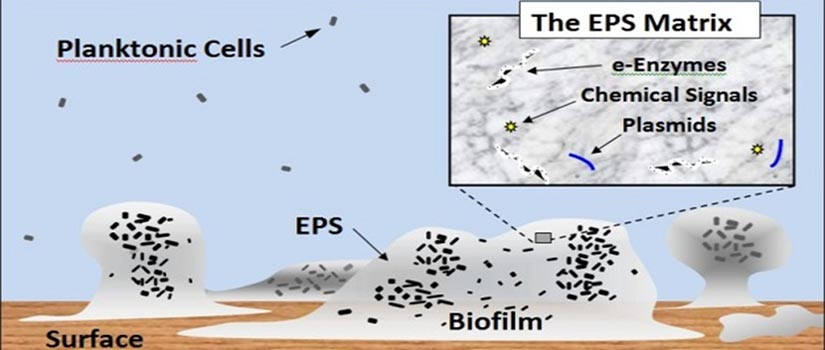
Bacteria are pivotal components of natural and engineered systems, including our own human bodies. We usually envision bacteria as individual cells, occurring here and there. In actuality, most bacteria occur as attached groups of cells called biofilms. As biofilms, cells collectively secrete a viscous, protective matrix of extracellular polymeric secretions (EPS), which surrounds and affords them capabilities not available to individual bacteria.
Biofilms occur when bacteria attach to surfaces (or tissues). Protective (EPS) polymers make biofilms resistant to antimicrobials, resulting in multi-billion dollar costs to health and society each year.
Biofilms occur virtually everywhere - having both positive and negative effects. The biofilm state provides bacteria the capability to act as coordinated groups; allowing them to communicate with each other and more efficiently adapt to stresses (e.g. UV, antibiotics, disinfectants), and even evade our immune system during infections. Microscopic biofilms, while barely visible to the eye, are recognized as an emerging area of importance to the environment and public health, one that imposes multi-billion dollar costs to health, industry, and society. They form resistant refugia for infectious bacteria, and are responsible for more than 70% of hospital acquired (nosocomial) infections, and contribute to the transfer of antibiotic-resistance. They also influence metal corrosion and outbreaks of disease during lapses in food security. In natural systems such as oceans, biofilms are commonplace, influencing marine snow formation, organic matter cycling, larval settlement processes, sediment stability, and optical properties of sediments. The biofilm matrix is a ‘sorptive sponge’ and site for the binding, transformation and trophic transfer of carbon, contaminants and nanoparticles. This exciting area of study requires multi-disciplinary approaches, and the efforts of colleagues and students in public health, chemistry, biology, engineering and medicine.